What's Unique About the Nanoscale?

Presented by

Scales


How “big” is “nano” ?
- The prefix “ nano” means “ one billionth”, or 10-9.
- A nanometer (nm) is one billionth of a meter.
- Nanometers are typically used to define dimensions between 0.1 nm (10-10 m) and 100 nm (10-7m).

Nanoscale
- Nanoscale: Nanoscale is a scale of measurements that uses “nano” or “10-9” as a multiplier to define its order of magnitude.
-
Nanoscale measurements
- Ex: Nanometers ->
nano
&
meter
- “ nano” defines the “scale” or “order of magnitude” of the unit of measurement.
- “ meter” is the unit of measurement for linear dimensions (e.g., length).
- It’s just like centimeter: where “centi” defines the order of magnitude 10-2.
- Ex: Nanometers ->
nano
&
meter
- Nanomaterials are talked about in nanometers, but nanoscale can apply to any unit of measure:
- Electrical current (nA: nanoamps).
- Voltage (nV: nanovolts).
- Time (ns: nanoseconds).
- Concentration (nM: nanomolar).
- Etc...
Visualize Nano
- We can try to visualize how small nano is by thinking about the various scales and powers of ten (10x).
- Try to relate them to things around you.
Look at the powers of ten from astronomical scale to atomic scale and beyond.
- in other words 10 26 to 10 -18.
Examine powers of ten in our own bodies.
Nanomaterials on a dimensional scale
- Typical nanomaterials in the 1-100 nm scale are shown on the lower side of the dimensional scale.
- The smallest nanomaterials are comparable in size to molecules, such as caffeine.
- Mid-size nanomaterials are comparable to biological macromolecules, such as nucleic acids and antibodies.
- Larger nanomaterials are comparable to viruses, and are still much smaller than any living entity (i.e., bacterial cells).

Nanomaterials vs Bulk Materials
- Nanomaterials offer unique properties compared to “bulk” materials.
- The uniqueness of nanomaterials and the ability to manipulate them is what makes nanotechnology so exciting and diverse.
- To understand just how unique nanomaterials are, let’s discuss characteristics of “non-nano” or bulk materials.
-
Bulk materials:
- the materials and items you are used to dealing with (larger than nanoscale).
- intrinsic properties are generally fixed, regardless of their shape, size, etc...
Bulk materials have fixed properties
- For example; the FIXED properties of gold in bulk.
- A piece of gold in a coin, a nugget, or part of a printed circuit board, will have the same properties (aka they are fixed).
- All bulk forms melt at same temp, have the same conductivity & density…



Bulk: different structure, different properties
- The only way to change the properties of bulk materials is to change its chemical composition or structure.
-
For instance, to change the conductivity of iron, you must make an alloy with other elements.
- You can see on the table how the conductivity changes as you combine iron with aluminum, silicon, carbon, etc.
-
Another example is if you want to change the color of a food dye molecule, you must completely change the chemical structure of the molecule.
Conductivity of iron-based alloys Color of food dye molecules




Nanomaterials do not have fixed properties
Nanomaterials behave differently from the corresponding bulk scale material.
At the nanoscale, everything is changeable!
- For a given nanomaterial, the properties are not fixed, but determined by the size and shape of the material.
- Not the chemical composition.
-
This is one of the most important concepts of nanotechnology.
- Characteristics of nanomaterials are dependent on structures, but strongly on size and shape.
- The same material can display radically different properties due to varying sizes, shapes.
Properties at the nanoscale: changing shape
All the properties (electrical, mechanical, chemical, etc.) are dependent of the size and shape of the material.
- To illustrate this concept, we will examine optical properties (color).
-
For example: Gold
- In BULK we know gold always has the same color no matter how it is shaped or the size of a gold object.




Properties at the nanoscale: changing shape
All the properties (electrical, mechanical, chemical, etc.) are dependent of the size and shape of the material.
-
For example: Gold
- At the NANOSCALE optical properties change as a function of size (diameter).
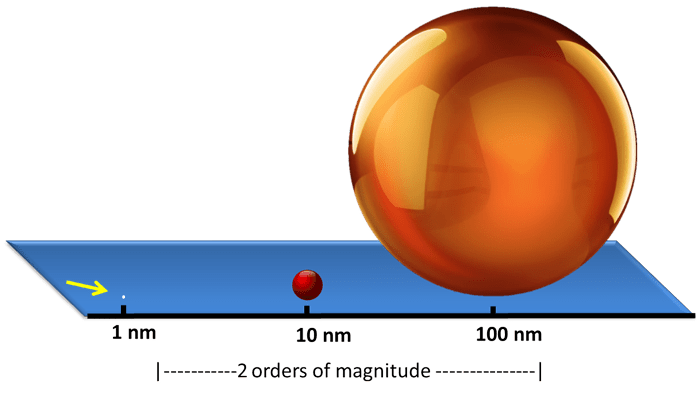
- Gold nanoparticles are easily made in the 1-100 nm range; here we have varying diameters of gold nanoparticles dispersed in solution.
- 100 nm gold nanoparticles are orange/brown, 10 nm gold nanoparticles are bright red, 1 nm nanoparticles are transparent.

Solutions on gold nanoparticles
Properties at the nanoscale: changing shape
- These are all gold atoms, but have very different properties!
- It is important to emphasize that the size and shape of these materials is more important than their chemical composition (which has not changed).
- Nanomaterial sizes range 2 orders of magnitude (1-100).



Bulk
Nano
Properties at the nanoscale: changing shape
-
Example: Cadmium Selenide (CdSe)
- In BULK (left) CdSe appears gray to the eye, like a gray slab/tombstone
- At the NANOSCALE (right), the color of CdSe color is dependent on its diameter
-
Very extreme example where a small variation in diameter results in a big change in color
- The difference between 3 and 5.5 nm is only 1.5 nm -- TINY TINY TINY! (** remember, a single nucleotide is 1 nm!)
Nano
Bulk


Cadmium Selenide
Cadmium Selenide Quantum Dots
Shape matters too!
Another look at how optical properties are changed, this time by shape.
- Here we examine nanoparticles of very similar sizes but with different crystalline structures -> therefore, different shapes.
Different shape configurations of nanoparticles.


Size & Shape-dependent
- Silver nanoparticles change color with different sizes and shapes.
- Great example of how size AND shape change the properties of a given nanomaterial.
- Illustrates chemical composition is not as important to change properties of nanomaterials.


Example: Al nanoparticles



- We took a look into the difference in optical properties between bulk and the nano equivalent of the same material.
-
Remember, there are many other properties that change with size and shape of nanomaterials.
- Not just optical properties/color.
- Aluminum for example…
Bulk Aluminum
Aluminum nanoparticles combust on contact with air!


Size and Shape-dependent
The chemical composition of the material is not as important as its size and shape for the resulting properties in nanomaterials.
- Nanomaterials can be tuned by various sizes or shapes
- “Custom made” to have specific, desired properties
- Now we can use materials with tailored properties for different applications
In-Class Assignment
Using your own words:
- List the following in order of increasing size: a buckyball, a soccer ball, a human red blood cell, a virus, an antibody, a molecule of insulin (a hormone), an E. coli cell, a molecule of methanol, and an 80-nm gold nanoparticle.
- Don’t know the size of something, look it up!
- Nanoscale refers to the magnitude 10x; x =?
- What kind of properties are fixed in bulk materials that may be different at the nanoscale? Give 2 examples.
- Properties of nanomaterials depend on their ___ and ___.

This lesson was presented by:
To learn more about nanotechnology, visit omninano.org

